As a high-quality choice for packaging materials such as food and beverages, tin plate sheet has high corrosion resistance and strength, which is inseparable from the protection of its surface tin coating. However, once the tin coating is damaged, can the tin plate sheet still maintain its corrosion resistance? Will the iron structure inside it be quickly oxidized and corroded?
This article will answer this question from multiple angles to help you understand the importance of the tin coating for tin plate sheet and its impact.
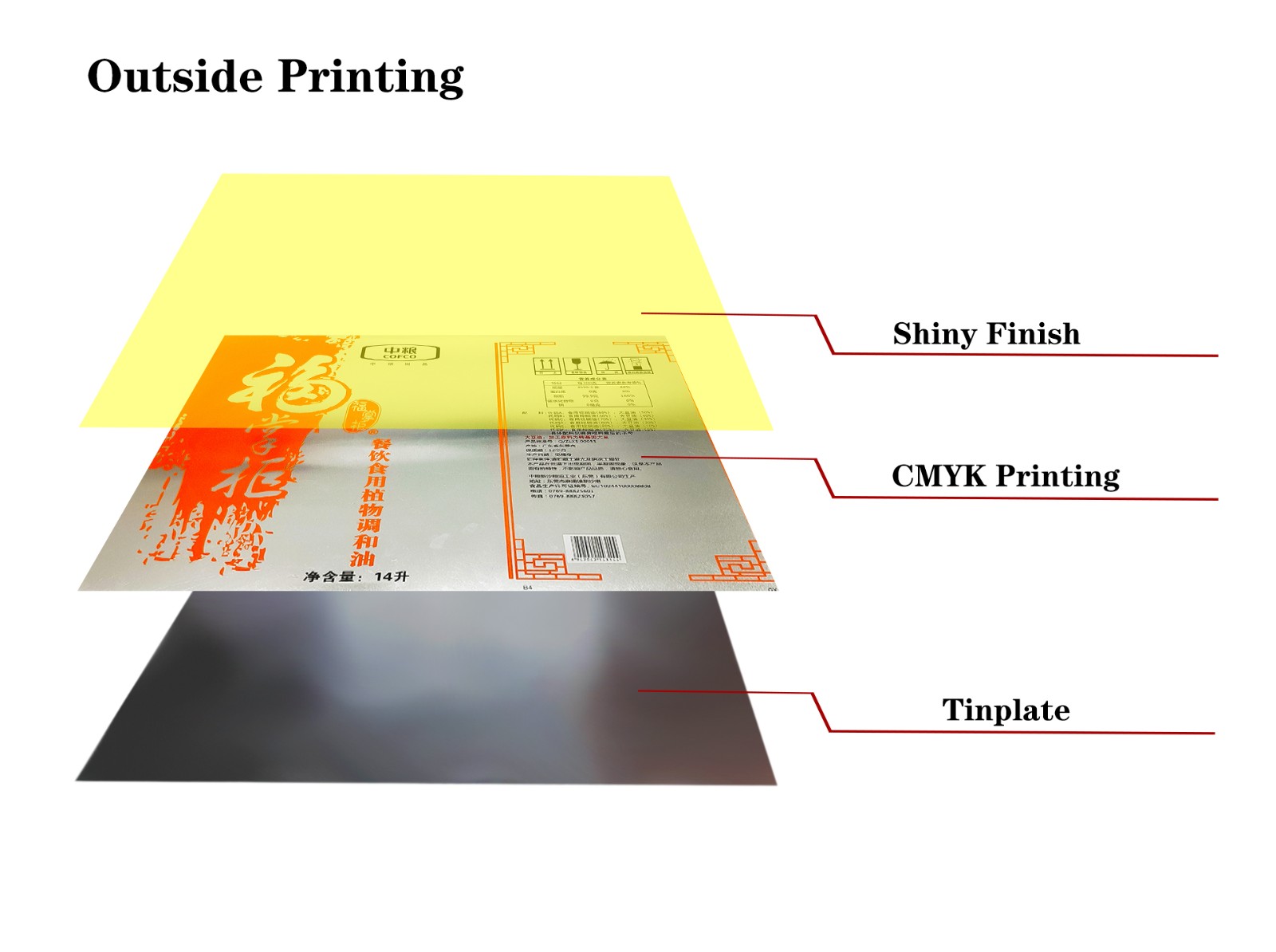
What is tin coating? What is its role?
1. What is the tin coating of tin plate sheet?
Tin plate sheet refers to a special steel with a layer of tin on the surface of the iron sheet. This process gives the iron sheet stronger corrosion resistance. The tin coating is usually electroplated or hot-dipped to evenly attach the tin layer to the iron sheet. Because the tin layer itself has good chemical inertness, it can prevent moisture and oxygen from entering the iron layer, making the tin plate sheet less likely to oxidize in moisture and air.
2. What is the protective role of the tin layer in tin plate sheet?
The key role of the tin layer is to isolate the iron sheet from direct contact with the outside world, thereby effectively slowing down the occurrence of corrosion. Tin is a less active metal that is not easy to react with oxygen, moisture or other chemical components in the air, thereby reducing the oxidation rate of the iron structure. Therefore, the role of the tin layer in the tin plate sheet is not only to decorate or add gloss, but more importantly to provide a solid protective barrier to extend the service life of the tin plate sheet.
What will happen to the tin plate sheet if the tin layer is damaged?
1. Is the area where the tin layer is missing prone to oxidation?
If the tin layer of the tin plate sheet is damaged or scratched, the exposed iron part will lose the protection of the tin layer and be directly exposed to the air. These exposed iron will quickly oxidize when encountering moisture and oxygen in the air to form rust. In contrast, the part covered by the tin layer can remain intact, and the undamaged area can still maintain good anti-corrosion properties.
2. Will local corrosion affect the entire iron sheet?
Partial damage to the tin plating layer may lead to accelerated local corrosion, especially in a humid environment. When the exposed iron part gradually rusts, the oxidation reaction will spread to the surrounding area, causing a wider range of corrosion problems. Especially when encountering acidic or saline substances, the corrosion rate will be further accelerated. Therefore, although it is only partially damaged, it is still possible to affect the durability and stability of the entire tin plate sheet.

Will the "galvanic corrosion" phenomenon occur in the tin plate sheet?
1. What is galvanic corrosion? Why does it happen on the tin plate sheet?
Galvanic corrosion refers to the potential difference that causes the more active metal to corrode faster when two metals with different electrode potentials are in contact and in an electrolyte environment. For tin plate sheet, when the tin plating layer is damaged and the iron is exposed, the moisture and pollutants in the air form an electrolyte environment. The electrode potential difference between tin and iron will cause local current to be generated, thus forming galvanic corrosion. Iron, as a more active metal, will become a "sacrificial anode" and accelerate corrosion, while the tin layer is relatively stable and the corrosion process is slower.
2. How much does galvanic corrosion affect the structure of tin plate sheet?
Galvanic corrosion can accelerate the corrosion rate of exposed iron parts, especially in humid or salty environments. The corrosion process will be further accelerated. As a result, at the damaged tin coating, the corrosion often penetrates deep into the iron structure, seriously affecting the physical strength and sealing of the entire iron sheet. Therefore, galvanic corrosion is not only a "rust" problem on the surface, it may directly weaken the overall structure of the tin plate sheet, and even cause contamination of the packaged items or failure of the packaging.
What factors will accelerate the corrosion after the tin coating is damaged?
1. The effect of environmental humidity on corrosion
A humid environment will significantly accelerate the corrosion rate at the damaged tin coating. Moisture will combine with oxygen in the air to form a water film on the iron surface, thereby enhancing the oxidation reaction. If the tin plate sheet packaging is placed in a high humidity environment, the iron part with damaged tin coating will oxidize faster to generate rust, causing the packaging to fail.
2. Will the acidic or alkaline environment aggravate corrosion?
Acidic or alkaline environments will also have a greater impact on damaged tin coatings. Acidic substances can directly react with iron to generate iron ions and release gas, which will corrode faster; while alkaline environment will cause electrochemical reaction, causing the exposed iron part to corrode rapidly. Therefore, when tin plate sheet comes into contact with acidic food, beverages or detergents, the corrosion rate of the damaged part will be significantly accelerated.
3. What effects will temperature change have?
Temperature is also an important factor affecting the corrosion process after the tin plate layer is damaged. Under high temperature conditions, the oxidation reaction speed is accelerated, and the oxidation process of the iron part will also be accelerated synchronously; when the temperature difference is large, the surface of the material may expand and contract, causing the tin plate layer to further crack and fall off, expanding the area and scope of corrosion.

How to protect the tin layer to extend the service life of tin plate sheet?
1. Can the correct storage environment delay corrosion?
Keeping the storage environment of tin plate sheet products dry, ventilated and light-proof can effectively delay the corrosion process. Avoid placing tin plate sheets in a humid, acidic, or alkaline environment, and reduce contact with moisture and oxygen, which can greatly protect the integrity of the tin layer and extend the service life of the tin plate sheet.
2. Is it necessary to regularly check and maintain the tin layer?
Regularly check the integrity of the tin layer, clean the stains and scratches on the surface in time, and take protective measures on the damaged parts before the corrosion spreads, such as applying anti-rust oil and sealing scratches. In addition, for more important storage items, especially food and medicine, it is recommended to choose high-quality tin plate sheet materials and pay attention to regular replacement of packaging to ensure the safety of the contents.
3. How to deal with tin plate sheet products with damaged tin layer?
When the tin layer has been damaged, it can be sealed with a transparent protective coating to prevent the exposed iron from being directly exposed to the air. If the scratch area is small, you can also try to repair it with a professional rust inhibitor. However, for tin plate sheet products with severe damage to the tin layer or large-area corrosion, it is recommended to replace them as soon as possible to prevent secondary contamination of the contents.
Buy Metal Packaging in Bulk with Competitive Pricing
Foshan Dekai Metal Packaging Co., Ltd. offers a comprehensive range of metal packaging products, including aerosol cans, tinplates, and custom-printed packaging. Our advanced production equipment ensures that we can handle large-scale orders while maintaining high-quality standards. We provide competitive pricing for bulk orders and offer flexible purchasing options, including wholesale deals and discounted rates.

